
About nanotech nano-robot pads! To target the brain
Translated from the Italian: https://igienistamentale.com/informazione/sui-tamponi-nano-robot-nanotech/
RESEARCHERS DESIGN SMALL MACHINES THAT DELIVER MEDICINE EFFICIENTLY
from Johns Hopkins University School of Medicine
Johns Hopkins Researchers Design Tiny Machines That
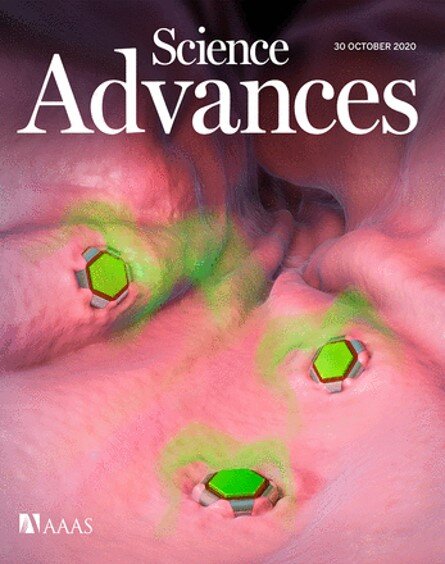
Deliver Drugs Efficiently A theragripper is the size of a speck of dust. This pad contains dozens of small devices. Credit: Johns Hopkins University.
Inspired by a parasitic worm digging its sharp teeth into its host’s gut, Johns Hopkins researchers have designed tiny star-shaped micro-devices that can attach to the intestinal mucosa and release drugs into the body.
David Gracias, Ph.D., professor at Johns Hopkins University Whiting School of Engineering, and Johns Hopkins gastroenterologist Florin M. Selaru, MD, director of the Johns Hopkins Inflammatory Bowel Disease Center, led a team of biomedical researchers and engineers. that designed and tested shape-changing microdevices that mimic the way parasitic hookworm attaches to an organism’s gut
Made of metal and thin film that changes shape and coated in a heat-sensitive paraffin wax, “theragrippers,” each approximately the size of a speck of powder, can potentially carry any drug and gradually release it into the body.
The team published the results of an animal study this week as a cover article in the journal Science Advances.
The gradual or sustained release of a drug is a long sought-after goal in medicine. Selaru explains that a problem with extended-release drugs is that they often make their way entirely through the gastrointestinal tract before they are finished dispensing the drugs.
“The normal constriction and relaxation of the muscles of the gastrointestinal tract make it impossible for the prolonged-release drugs to remain in the intestine long enough for the patient to receive the full dose,” says Selaru, who has worked with Gracias for more than 10 years. “We worked to solve this problem by designing these small drug transporters that can self-attach to the intestinal mucosa and maintain the drug load within the gastrointestinal tract for the desired duration.”
Thousands of theragrippers can be used in the gastrointestinal tract. When the paraffin coating on the forceps reaches the temperature inside the body, the devices self-close and snap to the colon wall. The closing action causes the tiny six-pronged devices to penetrate the mucosa and remain attached to the colon, where they are held and gradually release the payloads of the medicine into the body. Eventually, theragrippers lose their grip on the tissue and are expelled from the intestine via normal gastrointestinal muscle function.
Taken from the original research attachments
Thanks to the advances in biomedical engineering in recent years.
“We have seen the introduction of micro-fabricated dynamic smart devices that can be controlled by electrical or chemical signals,” he says. “But these clamps are so small that batteries, antennas and other components just don’t fit them.”
Theragrippers, says Gracias, doesn’t rely on electricity, wireless signals, or external controls. “Instead, they function like small compressed springs with a temperature-activated coating on the devices that release energy stored on its own at body temperature .”
Johns Hopkins researchers fabricated the devices with approximately 6,000 Theragrippers per 3-inch silicon wafer. In their animal experiments, they loaded a pain reliever drug onto the calipers. The researchers’ studies found that the animals given the theragrippers had higher concentrations of the analgesic in the bloodstream than the control group. The drug remained in the test subjects’ systems for nearly 12 hours versus two hours in the control group.
“Swarms of microscopic robots that can be injected” Tell Melinda Gates we can inject robots these days “
NANONEUROTHERAPEUTIC APPROACH FOR DIRECT DELIVERY FROM NOSE TO BRAIN
Expansion of affiliations
- PMID: 26057769
- DOI: 10.3109 / 03639045.2015.1052081
ABSTRACT
Background: Brain disorders remain the leading cause of disability in the world and represent more hospitalizations and prolonged care than almost all other diseases combined. Most drugs, proteins and peptides do not easily penetrate the brain due to the presence of the blood brain barrier (BBB), thus preventing the treatment of these conditions.
Objective: The focus has been on developing new and effective delivery systems to provide good bioavailability in the brain.
INTRANASAL DRUG DELIVERY SYSTEM BASED ON SAQUINAVIR MESILATE NANOEMULSION FOR BRAIN MIRAGE
Affiliations Hitendra S Mahajan 1, Milind S Mahajan , Pankaj P Nerkar , Anshuman Agrawal
Expand PMID: 24128122 DOI: 10.3109 / 10717544.2013.838014
EXTRACT
The central nervous system (CNS) is a prime immunological reservoir for providing sanctuary sites for HIV-1. Current anti-HIV drugs, although effective in reducing plasma viral levels, are unable to completely eradicate the virus from the body. The low permeability of HIV drugs across the blood brain barrier (BBB) leads to insufficient release. Therefore, for the treatment of neuro-AIDS it is necessary to develop new approaches that improve the delivery of anti-HIV drugs to the CNS. The aim of this study was to develop intranasal (NE) nanoemulsion for improved bioavailability and central nervous system targeting of saquinavir mesylate (SQVM). SQVM is a protease inhibitor which is a poorly soluble drug widely used as an antiretroviral drug, with oral bioavailability of approximately 4%. The spontaneous emulsification method was used to prepare the drug-loaded o / w nanoemulsion, characterized by droplet size, zeta potential, pH, drug content. In addition, ex vivo permeation studies were performed using sheep’s nasal mucosa. Optimized NE showed a significant increase in drug permeation rate compared to normal drug withdrawal (PDS). A cilia toxicity study on sheep nasal mucosa did not show any significant adverse effects of NE loaded with SQVM. Results from in vivo biodistribution studies show a higher drug with oral bioavailability of about 4%. The spontaneous emulsification method was used to prepare the drug-loaded o / w nanoemulsion, characterized by droplet size, zeta potential, pH, drug content. In addition, ex vivo permeation studies were performed using sheep’s nasal mucosa. Optimized NE showed a significant increase in drug permeation rate compared to normal drug withdrawal (PDS). A cilia toxicity study on sheep nasal mucosa did not show any significant adverse effects of NE loaded with SQVM. Results from in vivo biodistribution studies show a higher drug with oral bioavailability of about 4%. The spontaneous emulsification method was used to prepare the drug-loaded o / w nanoemulsion, characterized by droplet size, zeta potential, pH, drug content. In addition, ex vivo permeation studies were performed using sheep’s nasal mucosa. Optimized NE showed a significant increase in drug permeation rate compared to normal drug withdrawal (PDS). A cilia toxicity study on sheep nasal mucosa did not show any significant adverse effects of NE loaded with SQVM. Results from in vivo biodistribution studies show a higher drug characterized by droplet size, zeta potential, pH, drug content. In addition, ex vivo permeation studies were performed using sheep’s nasal mucosa. Optimized NE showed a significant increase in drug permeation rate compared to normal drug withdrawal (PDS). A cilia toxicity study on sheep nasal mucosa did not show any significant adverse effects of NE loaded with SQVM. Results from in vivo biodistribution studies show a higher drug characterized by droplet size, zeta potential, pH, drug content. In addition, ex vivo permeation studies were performed using sheep’s nasal mucosa. Optimized NE showed a significant increase in drug permeation rate compared to normal drug withdrawal (PDS). A cilia toxicity study on sheep nasal mucosa did not show any significant adverse effects of NE loaded with SQVM. Results from in vivo biodistribution studies show a higher drug Optimized NE showed a significant increase in drug permeation rate compared to normal drug withdrawal (PDS). A cilia toxicity study on sheep nasal mucosa did not show any significant adverse effects of NE loaded with SQVM. Results from in vivo biodistribution studies show a higher drug Optimized NE showed a significant increase in drug permeation rate compared to normal drug withdrawal (PDS). A cilia toxicity study on sheep nasal mucosa did not show any significant adverse effects of NE loaded with SQVM. Results from in vivo biodistribution studies show a higher drug
concentration in the brain after intranasal NE administration compared to intravenously administered PDS. The higher rate of drug targeting efficiency (% DTE) and direct nose-brain drug transport rate (% DTP) for optimized NE indicated effective CNS targeting of SQVM intranasally. Rat brain gamma scintigraphy imaging conclusively demonstrated drug transport into the CNS to a greater extent after intranasal administration as NE.
- Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K. Int J Pharm. 2008 Jun 24; 358 (1-2): 285-91. doi: 10.1016 / j.ijpharm.2008.03.029. Epub 2008 Mar 27. PMID: 18455333
- Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of sumatriptan. Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A. AAPS PharmSciTech. 2006 Jan 20; 7 (1): E8. doi: 10.1208 / pt070108. PMID: 16584167
- Nanoneurotherapeutics approach intended for direct nose to brain delivery. Md S, Mustafa G, Baboota S, Ali J. Drug Dev Ind Pharm. 2015; 41 (12): 1922-34. doi: 10.3109 / 03639045.2015.1052081. Epub 2015 Jun 9. PMID: 26057769 Review.
- Intranasal microemulsion for targeted nose to brain delivery in neurocysticercosis: Role of docosahexaenoic acid. Shinde RL, Bharkad GP, Devarajan PV. Eur J Pharm Biopharm. 2015 Oct; 96: 363-79. doi: 10.1016 / j.ejpb.2015.08.008. Epub 2015 Aug 28. PMID: 26318978
- Targeted drug delivery to the brain via intranasal nanoemulsion: Available proof of concept and existing challenges. Chatterjee B, Gorain B, Mohananaidu K, Sengupta P, Mandal UK, Choudhury H. Int J Pharm. 2019 Jun 30; 565: 258-268. doi: 10.1016 / j.ijpharm.2019.05.032. Epub 2019 May 13. PMID: 31095983 Review.
NANOPARTICLES AND NANOCOMPOSITES OF HYDROGEL FOR THE DELIVERY OF NASAL DRUGS / VACCINES
- Nanoparticles for nasal vaccination. Csaba N, Garcia-Fuentes M, Alonso MJ.Csaba N, et al. Adv Drug Deliv Rev. 2009 Feb 27; 61 (2): 140-57. doi: 10.1016 / j.addr.2008.09.005. Epub 2008 Dec 13. Adv Drug Deliv Rev. 2009.PMID: 19121350 Review.
- Nanoparticulate systems for nasal delivery of drugs: a real improvement over simple systems? Illum L. Illum LJ Pharm Sci. 2007 Mar; 96 (3): 473-83. doi: 10.1002 / jps.20718.J Pharm Sci. 2007.PMID: 17117404 Review.
- Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. Sharma S, Mukkur TK, Benson HA, Chen Y.Sharma S, et al. J Pharm Sci. 2009 Mar; 98 (3): 812-43. doi: 10.1002 / jps.21493.J Pharm Sci. 2009.PMID: 18661544 Review.
- Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. Mahajan HS, et al. Drug Deliv. 2014 Mar; 21 (2): 148-54. doi: 10.3109 / 10717544.2013.838014. Epub 2013 Oct 16 Drug Deliv. 2014.PMID: 24128122
- The application of mucoadhesive polymers in nasal drug delivery. Jiang L, Gao L, Wang X, Tang L, Ma J. Jiang L, et al. Drug Dev Ind Pharm. 2010 Mar; 36 (3): 323-36. doi: 10.1080 / 03639040903170750 Drug Dev Ind Pharm. 2010.PMID: 19735210 Review.
- Interleukin-4-loaded hydrogel scaffold regulates macrophages polarization to promote bone mesenchymal stem cells osteogenic differentiation via TGF-β1 / Smad pathway for repair of bone defect. Zhang J, Shi H, Zhang N, Hu L, Jing W, Pan J. Zhang J, et al. Cell Prolif. 2020 Oct; 53 (10): e12907. doi: 10.1111 / cpr.12907. Epub 2020 Sep 19.Cell Prolif. 2020.PMID: 32951298 Free PMC article.
- Application of Nanopharmaceutics for Flibanserin Brain Delivery Augmentation Via the Nasal Route. Ahmed OAA, Fahmy UA, Badr-Eldin SM, Aldawsari HM, Awan ZA, Asfour HZ, Kammoun AK, Caruso G, Caraci F, Alfarsi A, Al-Ghamdi RA, Al-Ghamdi RA, Alhakamy NA.Ahmed OAA, et al .Nanomaterials (Basel). 2020 Jun 29; 10 (7): 1270. doi: 10.3390 / nano10071270.Nanomaterials (Basel). 2020.PMID: 32610539 Free PMC article.
- Opioid antagonists as potential therapeutics for ischemic stroke. Peyravian N, Dikici E, Deo S, Toborek M, Daunert S. Peyravian N, et al. Prog Neurobiol. 2019 Nov; 182: 101679. doi: 10.1016 / j.pneurobio.2019.101679. Epub 2019 Aug 6.Prog Neurobiol. 2019.PMID: 31398359 Free PMC article. Review.
- Stimulus-responsive polymeric nanogels as smart drug delivery systems. Hajebi S, Rabiee N, Bagherzadeh M, Ahmadi S, Rabiee M, Roghani-Mamaqani H, Tahriri M, Tayebi L, Hamblin MR. Hajebi S, et al. Acta Biomater. 2019 Jul 1; 92: 1-18. doi: 10.1016 / j.actbio.2019.05.018. Epub 2019 May 13.Acta Biomater. 2019.PMID: 31096042 Free PMC article. Review.
- Preparation and Property Evaluation of Conductive Hydrogel Using Poly (Vinyl Alcohol) / Polyethylene Glycol / Graphene Oxide for Human Electrocardiogram Acquisition. Xiao X, Wu G, Zhou H, Qian K, Hu J. Xiao X, et al. Polymers (Basel). 2017 Jun 30; 9 (7): 259. doi: 10.3390 / polym9070259. Polymers (Basel). 2017.PMID: 30970936 Free PMC article.




https://humansarefree.com/2021/03/bombshell-public-health-england-admits-it-cannot-scientifically-prove-that-covid-19-is-contagious.html
https://humansarefree.com/2021/03/er-doctor-and-advanced-trauma-life-support-professor-i-have-never-seen-a-patient-sick-with-covid-19-we-are-being-deceived-and-manipulated.html